 Early demise due to illness or injury can shorten a mare’s reproductive career. Additionally, subfertility can also have a negative impact on a mare’s ability to produce a foal. There have been a multitude of advances in the past 20 years that have allowed for the preservation of genetics in mares. Techniques such as transvaginal ultrasound-guided oocyte aspiration (TVA) and intracytoplasmic sperm injection (ICSI) have allowed us to produce embryos in vitro, circumvent subfertility in mares, and preserve genetics from mares that have died.
Early demise due to illness or injury can shorten a mare’s reproductive career. Additionally, subfertility can also have a negative impact on a mare’s ability to produce a foal. There have been a multitude of advances in the past 20 years that have allowed for the preservation of genetics in mares. Techniques such as transvaginal ultrasound-guided oocyte aspiration (TVA) and intracytoplasmic sperm injection (ICSI) have allowed us to produce embryos in vitro, circumvent subfertility in mares, and preserve genetics from mares that have died.
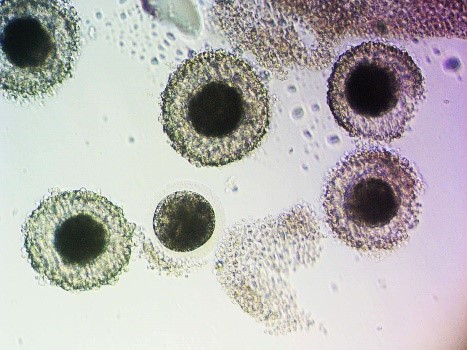
The first foal produced in vitro was born in 1996. This foal was produced from an in vivo matured oocyte from a donor mare, collected via TVA, fertilized in the laboratory with ICSI, cultured to form an embryo (blastocyst), and transferred back into the donor mare. This is a simplified explanation of the procedures that we perform to produce embryos in vitro. The first step to in vitro embryo production is the acquisition of oocytes (eggs) from the ovaries of a donor mare. This can be performed via scraping of follicles of harvested ovaries if a mare has had an untimely death, or TVA while she is still alive. In mares that have died, ovaries can be harvested and oocytes recovered from follicles present on the ovary. To obtain oocytes from live mares, transvaginal ultrasound-guided oocyte aspiration has become commonplace in many reproductive practices. This is an outpatient procedure performed under standing sedation with a mare restrained in the stocks. The ovaries are imaged with an ultrasound placed in the vagina and each follicle on the ovary is punctured, scraped, and rinsed with fluid several times. There are two types of follicles that can be aspirated during a TVA, dominant, gonadotropin-stimulated follicles and immature follicles. Oocyte recovery is higher from dominant follicles due to the loosening of the tight adherence between the oocyte and surrounding cumulus cells present in immature follicles. Typical recovery rates for a mature oocyte are 65-80%, whereas recovery rates for immature oocytes are 43-58%, however, rates can vary amongst laboratories [1-4]. Although recovery rates are higher for oocytes within mature follicles, typically only 1 oocyte is recovered, whereas all immature follicles can be aspirated and more oocytes recovered [2]. Once oocytes are acquired, they are processed and shipped to a laboratory where ICSI and embryo culture are performed.
 Recent research has shown that conventional in vitro fertilization can be repeatable in the horse, under certain conditions [5]. However, at this time, ICSI is still the most reliable method to produce embryos in vitro. When immature oocytes arrive at the ICSI laboratory, typically 24 hours after TVA was performed, they are placed into maturation culture media. Maturation rates vary between laboratories, but typically are around 50-60% [4,6,7]. Intracytoplasmic sperm injection involves the selection of 1 sperm and the injection of that sperm into a mature oocyte. The injected oocyte is then placed back into culture media and cultured for 7-10 days for embryo production. The embryo production rate also varies between laboratories but is about 20% [1, 2]. Any embryos produced can either be transferred directly into recipient mares or cryopreserved for transfer at a later date. Pregnancy rates from embryos transferred directly into recipients are not significantly different from those of conventionally acquired embryos, 60-75% [8]. Transvaginal ultrasound-guided oocyte aspiration can be performed at any time of a mare’s cycle and at any time of the year, as long as follicles are present on the ovaries [4, 9]. Aspirations can be performed every two weeks, with little impact on follicle numbers, mare fertility, and show schedule [4, 10, 11].
Recent research has shown that conventional in vitro fertilization can be repeatable in the horse, under certain conditions [5]. However, at this time, ICSI is still the most reliable method to produce embryos in vitro. When immature oocytes arrive at the ICSI laboratory, typically 24 hours after TVA was performed, they are placed into maturation culture media. Maturation rates vary between laboratories, but typically are around 50-60% [4,6,7]. Intracytoplasmic sperm injection involves the selection of 1 sperm and the injection of that sperm into a mature oocyte. The injected oocyte is then placed back into culture media and cultured for 7-10 days for embryo production. The embryo production rate also varies between laboratories but is about 20% [1, 2]. Any embryos produced can either be transferred directly into recipient mares or cryopreserved for transfer at a later date. Pregnancy rates from embryos transferred directly into recipients are not significantly different from those of conventionally acquired embryos, 60-75% [8]. Transvaginal ultrasound-guided oocyte aspiration can be performed at any time of a mare’s cycle and at any time of the year, as long as follicles are present on the ovaries [4, 9]. Aspirations can be performed every two weeks, with little impact on follicle numbers, mare fertility, and show schedule [4, 10, 11].
Transvaginal ultrasound-guided oocyte aspiration and ICSI allow for the production of embryos from mares with reproductive abnormalities of the cervix, uterus, and oviducts or those that have been unsuccessful in conventional breeding or embryo transfer programs. Recent data has shown that older mares had higher reproductive efficiency with TVA-ICSI versus natural breeding, artificial insemination, and embryo flush and transfer [8]. Also, semen from stallions with poor fertility or low sperm reserves can be utilized to produce foals with these techniques. The field of equine reproduction is rapidly changing and new assisted reproductive techniques allowing for the genetic preservation of mares will continue to advance within the next 10 years.
You may also be interested in the following articles:
Intrafollicular Oocyte Transfer in the Mare
Unexplained Subfertility in Broodmares
Breeding Soundness Exam of the Mare
______________________________________________________________________________________________
[1] Hinrichs K. In vitro production of equine embryos: state of the art. Reprod Domest Anim. 2010;45 Suppl 2:3-8.
[2] Hinrichs K. Assisted reproductive techniques in mares. Reproduction in Domestic Animals. 2018;53:4-13.
[3] Hinrichs K, Kenney DF, Kenney RM. Aspiration of oocytes from mature and immature preovulatory follicles in the mare. Theriogenology. 1990;34:107-12.
[4] Jacobson CC, Choi YH, Hayden SS, Hinrichs K. Recovery of mare oocytes on a fixed biweekly schedule, and resulting blastocyst formation after intracytoplasmic sperm injection. Theriogenology. 2010;73:1116-26.
[5] Felix MR, Turner RM, Dobbie T, Hinrichs K. Successful in vitro fertilization in the horse: production of blastocysts and birth od foals after prolonged sperm incubation for capacitaton. Biology of Reproduction. 2022;107,6:1551-1564.
[6]Colleoni S, Barbacini S, Necchi D, Duchi R, Lazzari G, Galli C. Application of ovum pick-up, intracytoplasmic sperm injection and embryo culture in equine practice. Proc Am Assoc Equine Pract. 2007;53:554-9.
[7] Bøgh IB, Bézard J, Duchamp G, Baltsen M, Gérard N, Daels P, et al. Pure preovulatory follicular fluid promotes in vitro maturation of in vivo aspirated equine oocytes. Theriogenology. 2002;57:1765-79.
[8] Stout T, Cuervo-Arango J, De Ruijter-Villani M, Claes A. Oocyte collection in the mare; efficiency of ovum pick-up, and techniques for shipping ovaries and/or oocytes. Leipziger Blaue Hefte.277.
[9] Choi Y-H, Velez IC, Macías-García B, Riera FL, Ballard CS, Hinrichs K. Effect of clinically-related factors on in vitro blastocyst development after equine ICSI. Theriogenology. 2016;85:1289-96.
[10] Velez IC, Arnold C, Jacobson CC, Norris JD, Choi YH, Edwards JF, et al. Effects of repeated transvaginal aspiration of immature follicles on mare health and ovarian status. Equine Veterinary Journal. 2012;44:78-83.
[11] Mari G, Barbara M, Eleonora I, Stefano B. Fertility in the mare after repeated transvaginal ultrasound-guided aspirations. Animal Reproduction Science. 2005;88:299-308.


Log in to join the conversation.