 I have always been fascinated by the exquisite design of biological systems. The more we humans understand about biology, the more we realize we don’t know. The process of mammalian fertilization is one of these complex biological systems that in nature requires the proper coordination of so many factors ranging from the behavior of male and female to biochemical changes at the cellular and molecular level. Defined as: “A process in sexual reproduction that involves the union of male (sperm) and female (ovum) gametes (each with a single, haploid set of chromosomes) to produce a diploid zygote”, fertilization requires that functionally viable sperm, at the right stage of maturity, are present in the oviduct of the mare during a brief window of time when a functionally viable oocyte is present.
I have always been fascinated by the exquisite design of biological systems. The more we humans understand about biology, the more we realize we don’t know. The process of mammalian fertilization is one of these complex biological systems that in nature requires the proper coordination of so many factors ranging from the behavior of male and female to biochemical changes at the cellular and molecular level. Defined as: “A process in sexual reproduction that involves the union of male (sperm) and female (ovum) gametes (each with a single, haploid set of chromosomes) to produce a diploid zygote”, fertilization requires that functionally viable sperm, at the right stage of maturity, are present in the oviduct of the mare during a brief window of time when a functionally viable oocyte is present.
Spermatogenesis
Spermatogenesis (the process of sperm formation in the testes) is an ongoing process with newly formed sperm being produced constantly during the sexually mature lifespan of the male. In the stallion, spermatogenesis takes approximately 57 days from start to finish. Further sperm maturation occurs during transit of the sperm through the epididymis where motility and fertilizing capacity is acquired. Mature sperm are then stored in the tail of the epididymis and the ampulla until ejaculation. At ejaculation, the sperm are exposed to factors in fluid from the accessory sex glands that are important in protecting the sperm from the immune system of the mare. Once ejaculated into the mare’s uterus the sperm undergo further changes in response to specific signals from the female reproductive tract. These physical and biochemical changes (capacitation and the acrosome reaction) are required for the sperm to bind to and penetrate the oocyte and initiate fertilization. Once the changes associated with capacitation and the acrosome reaction occur, the sperm have a finite period to encounter a mature oocyte after which they rapidly die and are no longer able to participate in fertilization. So, successful fertilization requires that there are sufficient “functionally viable sperm” that have survived the transit through the female reproductive system, that are at the right stage of maturation at the right time and in the right place (oviduct) when a mature oocyte is present. (see article “It Only Takes One…Right?”)
Prolonging the Lifespan of Sperm
The use of artificial insemination (AI) with cooled and frozen semen is our attempt to remove some of the natural barriers to successful fertilization such as geographical distance between sire and dam or asynchrony of optimum time for mating. AI is also used to improve efficiency of breeding by allowing for multiple mares to be inseminated from a single ejaculate. AI with frozen semen allows for International distribution of superior genetics and the possibility to access sperm from exceptional sires that are no longer fertile or even deceased.
To prolong the lifespan of sperm after ejaculation and interrupt the normal timing of sperm maturation and death, semen processing laboratories will slow down or completely halt metabolism by cooling or freezing the sperm. Without proper processing, cooling and/or freezing of sperm causes lethal damage to the cells. In this article, we will discuss the major causes of sperm damage during cryopreservation and what steps are taken to minimize that damage.
A solution consists of a solute and a solvent. The solute is the substance that is dissolved in the solvent. For example, in a saline solution, salt is the solute dissolved in water as the solvent. Ejaculated semen consists of spermatozoa in a suspension of seminal fluids. In an isosmotic (or isotonic) physiological state, the concentration of solutes dissolved in the seminal fluid is equal to the concentration of solutes within the cell. The membranes that surround the spermatozoa can allow for the movement of water and ions across the cell membrane to maintain this equilibrium and keep the osmotic pressure equal across the cell membrane.
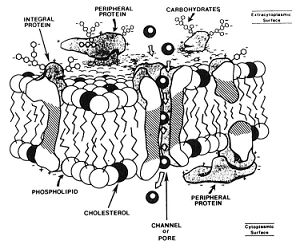
In a suspension of cells, a solution that contains a concentration of solutes higher than that inside of the cell is hypertonic and one that has a lower concentration of solutes is hypotonic. The membranes that surround compartments of the sperm are comprised of a bilayer of various lipids and proteins (diagram above left) that are arranged to serve certain functions including transport of molecules, ions and water between the inside and outside of the cells. At body temperature, these lipid membranes are fluid and the proteins can move laterally within the membrane. At reduced temperatures, the lipids undergo phase changes from liquid to gel to solid states and the arrangement of membrane components can be altered, leading to changes in permeability, premature capacitation and eventual cell death.
Sperm Damage During Cryopreservation
The traditional theory of sperm damage during cryopreservation is the two-factor hypothesis. In this theory, the two major factors contributing to damage are:
- intracellular ice formation resulting from rapid cooling and
- solution effects injury resulting from prolonged exposure of the cells to extreme hyperosmotic conditions if cooled too slowly
As the solution is cooled slowly below the freezing point the first thing to happen is ice nucleation in the extracellular solution. Microcrystals of pure water freeze outside the cell which results in a higher concentration of solutes outside the cell as compared to inside. To balance the osmotic gradient, water diffuses from inside to outside the cell causing partial dehydration of the cell and subsequent reduction in cell volume. As the temperature is further reduced, the concentration of solutes increases, the freezing point of the solution decreases, more dehydration occurs and the cell continues to shrink. This continues until the solution can no longer exist in a liquid state and the remaining solution crystalizes with the solutes in place. Prolonged exposure to these high solute concentrations causes denaturation of macromolecules and irreversible membrane damage.
 Conversely, if the cooling is too rapid then the water doesn’t have time to move outside the cell and large intracellular ice crystals form which can physically damage the cells.
Conversely, if the cooling is too rapid then the water doesn’t have time to move outside the cell and large intracellular ice crystals form which can physically damage the cells.
The ideal cooling curve (right) to minimize cell damage is one which is slow enough to allow for partial dehydration of the cell and avoid large intracellular ice crystal formation but fast enough to minimize damage from solution effects.
To minimize damage, sperm are suspended in extenders that contain a number of components designed to protect the sperm during cooling and freezing. In addition to salts, buffers, water and antibiotics both non-penetrating (sugars) and penetrating (glycerol, DMSO, ethylene glycol, amides) cryoprotective agents (CPA’s) are included. Freezing extenders also typically include sources of lipid and lipoprotein from egg yolk and milk. The low-density lipoproteins in egg yolk and the casein in milk are thought to protect the sperm from cooling damage and may play a role in membrane repair. Penetrating CPA’s like glycerol, ethylene glycol or the amides protect sperm by lowering the temperature at which the cells are exposed to critically high salt concentrations while non-penetrating CPA’s like lactose protect the cells through osmotic properties that promote rapid dehydration of cells.
However, evidence from recent studies with human and horse sperm indicate that with the cooling rates typically used for cryopreservation of sperm there is little or no formation of intracellular ice. Therefore, the major source of damage may be primarily due to osmotic stress caused by extracellular ice formation and the resulting changes in relative cell volume during freezing and thawing.
Let’s consider the changes in cell volume (see graph below) that likely occur during a typical freeze-thaw cycle. For horse semen cryopreservation protocols:
- Sperm are first suspended in an isotonic solution during which there is no change in cell volume.
- Following centrifugation to remove most of the seminal plasma, sperm are suspended in a hypertonic freezing extender that contains both penetrating and non-penetrating CPA’s.
- As a result, the water moves out across the cell membrane to balance the osmotic gradient and the cell volume is decreased temporarily until the penetrating CPA diffuses into the cell and equilibrium is reached at which point the cell returns to its original volume.
- Next, as the suspension is cooled and pure water begins to crystalize outside the cell the solute concentration outside the cell increases and once again water moves out of the cell across the plasma membrane resulting in dehydration and a reduction in cell volume. This continues until the solution freezes and the sperm are held in that state at -196C (liquid nitrogen temperature) until thawed.
- Upon thawing the external ice begins to melt, the solute concentration decreases and water moves back into the cell returning it to its original volume.
 If the sperm are maintained after thawing in the freezing extender containing the same concentration of penetrating CPA then the original cell volume is maintained. However, when those sperm are placed into an environment that does not contain penetrating CPA (such as a diluent for motility evaluation or the mare’s uterus following insemination), water will move into the cell to balance the concentration of CPA. All penetrating CPA’s have a permeability across the plasma membrane that is considerably slower than water therefore this influx of water causes a swelling of the sperm and increased cell volume until the CPA diffuses out of the cell. Large molecular weight CPA’s such as glycerol diffuse across the membrane much more slowly than low molecular weight CPA’s such as ethylene glycol, and amides.
If the sperm are maintained after thawing in the freezing extender containing the same concentration of penetrating CPA then the original cell volume is maintained. However, when those sperm are placed into an environment that does not contain penetrating CPA (such as a diluent for motility evaluation or the mare’s uterus following insemination), water will move into the cell to balance the concentration of CPA. All penetrating CPA’s have a permeability across the plasma membrane that is considerably slower than water therefore this influx of water causes a swelling of the sperm and increased cell volume until the CPA diffuses out of the cell. Large molecular weight CPA’s such as glycerol diffuse across the membrane much more slowly than low molecular weight CPA’s such as ethylene glycol, and amides.
If the volume change exceeds the osmotic tolerance limits of the membranes then they will be irreversibly damaged and cause cell death. Species differ in the susceptibility of their sperm to damage due to cold shock and cryopreservation. These species-specific differences are thought to be related to the biochemical structure of the plasma membranes, specifically the cholesterol:phospholipid ratios, fatty acid content and membrane fluidity. It is believed that these differences are likely responsible for the differences in osmotic stress tolerance seen between species whose sperm survive cryopreservation well versus those that do not.
Male to Male Variation Within a Species
In addition to this species-specific variability, a well-documented inherent variation exists between individual males of many species in the ability of their sperm to withstand the stresses associated with freezing and thawing (cryotolerance). This male to male variation is especially evident in stallions. In dairy cattle, bulls have been selected by the AI industry for more than 50 years based on the ability of their sperm to withstand the stresses of standard cryopreservation protocols. This selection has led to an increasingly uniform and positive response to cryopreservation. Studies on membrane fluidity and osmotic stress tolerance have demonstrated that bull sperm have a much greater tolerance for exposure to hypertonic conditions than stallion sperm and that there was a 3-fold greater variance in osmotic stress tolerance between individual stallions than between individual bulls. Studies with boar sperm and human sperm have also revealed significant male to male variation in plasma membrane composition and some correlations have been found between cholesterol to phospholipid ratios, membrane fluidity, fatty acid content and response to cryopreservation. Further evidence for the relationship between membrane composition and cryosurvival comes from experiments with 4 different strains of mouse sperm that vary significantly in their cholesterol:phospholipid ratio. The percentage of motile sperm after thawing was directly correlated with the cholesterol:phospholipid ratio. The researchers were also able to dramatically improve cryosurvival in the low cholesterol strain by increasing the cholesterol content of the sperm membranes with cholesterol loaded cyclodextrins.
The SBS Difference
 To date there is no single universal cryopreservation protocol that is optimum for semen from all stallions and use of a single protocol (extender, cooling rate, etc.) has led to the belief that stallions can be grouped into “good” and “bad” freezers based on post-thaw evaluation of semen frozen using a single common protocol. The SBS System for freezing stallion semen is based on the belief that semen from a large percentage of stallions in the population can be frozen successfully if an effort is made to customize cryopreservation protocols to identify optimum conditions for each individual stallion.
To date there is no single universal cryopreservation protocol that is optimum for semen from all stallions and use of a single protocol (extender, cooling rate, etc.) has led to the belief that stallions can be grouped into “good” and “bad” freezers based on post-thaw evaluation of semen frozen using a single common protocol. The SBS System for freezing stallion semen is based on the belief that semen from a large percentage of stallions in the population can be frozen successfully if an effort is made to customize cryopreservation protocols to identify optimum conditions for each individual stallion.
Our goals are to:
- Produce the highest quality frozen semen from every individual stallion, not just accepting what appears to be adequate based on results from a single standard protocol and
- Identify conditions for genetically desirable individual stallions deemed to be “poor freezers” that allows them to be included in commercial frozen semen breeding programs.
Excluding a champion performance stallion from a commercial frozen semen breeding program based on results from a single cryopreservation protocol is not acceptable if frozen semen is to be a significant tool in modern horse breeding.
The SBS approach employs multiple protocols that are designed to determine the optimum procedure for maximum fertility of frozen semen from each individual stallion. Standard practice for all new stallions is to perform 1 or 2 split-ejaculate test freeze procedures using a variety of extenders that contain different sources and amounts of lipids, proteins, sugars and various penetrating and non-penetrating cryoprotectants designed to control damaging cell volume excursions during freezing and thawing.
Conclusion
A great deal of progress has been made in the fundamental understanding of how sperm from many species are compromised during cryopreservation and this information has led to development of new extenders and protocols that have improved the results of frozen semen inseminations. Today, with improved freezing protocols leading to better quality frozen semen and a greater understanding of how to manage mares for insemination with frozen semen, this technology is more widespread than ever in horse breeding. And given the stress that we humans have placed upon nature’s exquisitely developed system of reproduction, one can marvel at how successful this technology has been so far.


Log in to join the conversation.